The Medicines Patent Pool (MPP) announced on January 20 that it has signed agreements with 27 generic manufacturing companies for the manufacturing of the oral COVID-19 antiviral medication molnupiravir and supply in 105 low- and-middle-income countries (LMICs). The sublicence agreements are the result of the voluntary licensing agreement signed by MPP and MSD, a trade name of Merck & Co., Inc., in October 2021 to facilitate affordable global access for molnupiravir, that MSD is developing in partnership with Ridgeback Biotherapeutics.
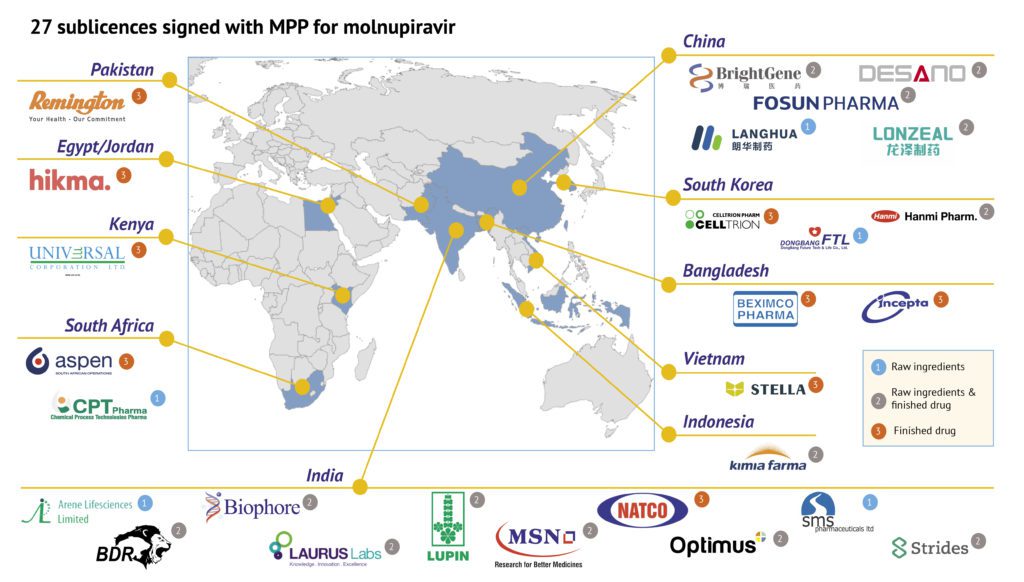
Five Chinese drugmakers Fosun Pharma (复兴医药), BrightGene Bio-Medical Technology Co., Ltd. (博瑞生物医药(苏州)股份有限公司), Zhejiang LangHua Pharmaceutical Co., Ltd. (浙江朗华制药有限公司), Shijiazhuang Lonzeal pharmaceuticals Co., Ltd. (石家庄龙泽制药股份有限公司), and Shanghai Desano Chemical Pharmaceutical Co., Ltd. (上海迪赛诺化学制药) are among in the licensed companies, while molnupiravir will not supplied in China under this agreement, which is not defined as a low-income country.
Under the deal, negotiated by the U.N.-backed Medicines Patent Pool (MPP) with Merck, the U.S. company will not receive royalties for the sale of the low-cost version of the pill while the pandemic continues.
The non-exclusive sublicences allow generic manufacturers to produce the raw ingredients for molnupiravir and/or the finished drug itself. The companies that were offered the sublicence successfully demonstrated their ability to meet MPP’s requirements related to production capacity, regulatory compliance, and the ability to meet international standards for quality-assured medicines. Five companies will focus on producing the raw ingredients, 13 companies will produce both raw ingredient and the finished drug and 9 companies will produce the finished drug. The companies span 11 countries, Bangladesh, China, Egypt/Jordan, India, Indonesia, Kenya, Pakistan, South Africa, South Korea, and Vietnam.
|
Copyright © 2003-2018 China Intellectual Property Magazine,All rights Reserved . www.chinaipmagazine.com 京ICP备09051062号 |
|
|


